Indium-111 White Blood Cell Imaging:
Chemistry and Pharmacology:
Indium-111 (valence +3) is cyclotron produced by the proton irradiation of 112Cd-enriched targets according to the reaction 112Cd(p,2n)111In [39]. Indium-111 has a physical half-life of 67.3 hours and it decays by electron capture with gamma emissions of 173 and 247 keV to stable 111Cd [39]. Indium-111 oxine is a highly lipophilic ligand which diffuses across leukocyte membranes. Once inside the cell, it dissociates into oxine (8-hydroxyquinoline, which diffuses out of the cell [7]) and In-111 which is retained in the cytoplasm by binding to intracellular proteins. A white blood cell rich fraction is separated from the other blood components by both gravity sedimentation and centrifugation. The percentage of contamination with other cell types is very low. The resultant white cell rich pellet is resuspended in saline for labeling. Indium oxine cannot be used to label the cells if they are still suspended in plasma as 90% of the radiopharmaceutical binds to transferrin rapidly and irreversibly. (There was some controversy as to whether WBC's maintain adequate function after they have been removed from plasma for labeling although years of clinical experience would argue to the contrary.) All cellular blood components are labeled including lymphocytes, platelets, and red blood cells in proportion to their total available cell membrane surface area. Although a mixed population of cells is labeled, the majority are neutrophils- hence, the procedure is most useful for detecting neutrophil-mediated inflammatory processes [32]. The critical organ is the spleen which receives approximately 9 to 14 rads per 0.5 mCi dose (but as high as 60 rads to the spleen in an infant [29]). An individual neutrophil receives about 750-1500 [7] rads from a 0.5 mCi dose of 111-In. This dose has not been shown to have any adverse effect on leukocyte migration or chemotaxis. Lymphocytes incidentally labeled with 111-In in mixed leukocyte preparations are killed by the radiation and pose no long term cancer risks. If there is fragmentation of the labeled white blood cells, indium- transferrin complexes are formed and more activity will be seen within the liver and bone marrow.
Technique:
Approximately 60cc of the patient's blood is removed. After gravity sedimentation to remove the majority of red cells, centrifugation is performed to obtain a white blood cell rich pellet. The plasma is set aside for later resuspension of the cells after labeling is complete. For an adult, 500uCi of 111In is used when labeling the WBC's. Incubation of the cells and the 111In-oxine takes place at room temperature for approximately 30 minutes, during which labeling occurs. It is CRUCIAL that the patient receive his/her own cells back. Many clinics therefore require that the person who draws the blood for labeling also re-inject the labeled cells. Following injection, the labeled neutrophils are cleared from the circulation rapidly with a half-time of 6 to 7 hours. Although transient pulmonary activity is seen up to about 4 hours following injection, by 24 hours the normal distribution of activity is to the liver (10-20%), the spleen (20-40%) which has the highest intensity, and the bone marrow (30-60%) [1]. The pulmonary activity is due to the cells' having contacted the extracorporeal glass surfaces during labeling that results in cellular activation which impedes passage through the pulmonary vasculature [42]. As a result they tend to marginate in the lungs as is the case in the transient neutropenia of dialysis. 111-In WBC's may also aggregate, and upon injection, become trapped in the capillary bed of the lungs producing one or more pulmonary foci. Occasionally significant blood pool activity remains- this may indicate that a large number of erythrocytes have also been labeled [7].
Images are typically performed 18-24 hours following injection of the labeled WBC's because early imaging at 4 hours will miss two-thirds of the lesions detected on later images. However, it is critical to obtain early (1-4 hour) images when evaluating patients for inflammatory bowel disease or those suspected of possible ischemic bowel disease. Occasionally, 48 hour delayed images may be necessary due to prolonged circulation of labeled cells in about 10% of cases. Activity at sites of intravenous catheters, NG tubes, drainage tubes, colostomies, and tracheostomies generally only represents colonization and NOT infection, unless large or markedly intense labeled cell accumulations are seen. Similarly, uninfected post-surgical wounds will also commonly show mild uptake for the first 10 days. Injection of the labeled cells through central lines should be avoided if possible, as activity may adhere to the line itself.
Donor WBC's may be used in neutropenic patients (i.e.: WBC count
less than 2000). Another approach in borderline neutropenic
individuals includes doubling the amount of blood removed. Donor
cells need only be ABO compatible and are best obtained from a
recently HIV negative, Hepatitis negative/vaccinated health care
provider. Informed consent is required, although standard type and
cross match should be performed. Infections which do not generate
a significant leukocyte response (i.e.: chronic, parasitic, or
fungal infection), may best be evaluated with other modalities
such as 67Ga.
The relative radiation dose from the In-111 WBC examination in adults is between 4.0-6.7 mSv (and 6.8-10.2 mSv when combined with dual-isotope Tc-sulfur colloid imaging) [44]. High splenic radiation doses (up to 60 rad in an infant) limit the use of 111In labeled white cells in the imaging of children and if necessary, 99mTc-HMPAO cells may be used.
In-111 WBC Imaging in Acute Infection:
(Sensitivity: 90%, Specificity: 90%- specificity is much higher than 67Ga imaging [65%]).
111In labeled leukocytes localize at sites of infection through diapedesis, chemotaxis, and enhanced vascular permeability. In rats, about 10% of the circulating WBC's are recruited to a site of infection by 24 hours.
Datz and Thorne [2] evaluated the effect of antibiotics on the 111In WBC scan and found no significant difference in the diagnostic utility of 111In WBC imaging (sensitivity was 89% in patients on antibiotics, compared to 92% in patients not on therapy). Other conditions which may theoretically affect leukocyte chemotaxis include uremia, hemodialysis, hyperalimentation, steroid use, and chemotherapy agents, however, no significant effect on the sensitivity of leukocyte imaging has been demonstrated in these conditions either [3]. 111In WBC's are probably the agent of choice for imaging suspected sites of abdomen infection, while Gallium is preferable for detecting pulmonary pathology and in the setting of FUO.
Factors which may reduce the sensitivity of 111In WBC imaging include:
- Neutropenic patient
- Adjacent soft tissue infection or septic joint
- Liver/Spleen activity may obscure site of infection
- Inadequate blood withdrawn for labeling (esp. children)
- Marrow hyperplasia (Sickle cell, Thalassemia): May have asymmetries which can mimic osteomyelitis
- Uptake in inguinal/iliac reactive adenopathy
Remember: Antibiotic therapy, Steroids, Hyperglycemia, Uremia, and Hyperalimentation have not been shown to reduce the sensitivity of 111In WBC imaging.
In-111 WBC Imaging in Chronic Infection:
Some authors feel that there is no significant difference in the sensitivity of labeled leukocytes for the detection of either acute or chronic non-skeletal infection. The sensitivity is about 90% for acute infections, and about 85% for chronic infections [4]. The excellent detection of chronic infections may be because they still contain and attract neutrophils. Additionally, some labeled lymphocytes may survive and localize to these sites [3]. However, other authors have questioned the sensitivity for the detection of infections that are not neutrophil mediated (such as PCP pneumonia and TB) [40]. Datz et al found no loss of sensitivity with chronic infections, while others suggest sensitivity for detecting chronic infection may range from 60 to 90%.
Osteomyelitis Imaging:
Osteomyelitis can occur from three potential routes of infection- hematogenous (accounts for 20% of adult cases of osteomyelitis, but is the most common route in pediatric patients), direct innoculation, and local extension from contiguous infection [41]. The organism most commonly responsible for hematogenous osteomyelitis is S. aureus. Group B streptococcal infection is seen more commonly in neonates. In drug addicts and nosocomial infections gram negative bacteria such as Pseudomonas and Klebsiella are commonly encountered. E. coli is usually the cause of spinal osteomyelitis following urinary tract infection or instrumentation. In patients with sickle cell anemia, infections with S. aureus and Salmonella are common.
Overall, osteomyelitis in chidlren is rare (6 cases per 1000 admissions) [41]. The most common organism is S. aureus, followed by the respiratory pathogens S. pneumoniae, S. pyogenes, and Kingella kingae [43]. Osteomyelitis primarily affects young children, with half of all pediatric cases in children under 5 years of age [43]. Boys are affected twice as often as girls [43]. In children osteomyelitis most commonly occurs in the metaphyseal region of long bones [41,43]. In young children, the metaphyseal spongiosa contains abundant blood vessels that end in capillary loops with leaky endothelium and turbulent slow flow which favor hematogenous bacterial deposition [41,43]. Epiphyseal and joint involvement is common in children under 18 months of age due to the presence of transphyseal vessels. In older children the growth plate prevents the spread of infection into the epiphysis. Metaphyseal equivalents with increased risk for infection can be seen in flat bones, such as the pelvis, adjacent to the sacroiliac joints, symphysis pubis, ischiopubic synchondroses, or triradiate cartilage. Other areas include the periphery of round bones such as the talus, and the periphery of secondary centers of ossification [43]. In neonates with osteomyelitis, systemic disturbances may be mild or absent. Detection is therefore frequently delayed, and involvement can be multicentric. Pain, limp, or refusal to bear weight are often the only symptom in older children. Fever and focal tenderness can be seen [43]. About one-third of children have a leukocytosis, but if both the ESR and C reactive protein values are abnormally elevated, the sensitivity for infection in 98% [43]. Blood cultures are positive 40% of less of cases, while bone, joint, or soft tissue cultures have a higher yield in the range of 70% [5,43].
With acute osteomyelitis plain radiographs may demonstrate soft tissue swelling, a periosteal reaction, cortical irregularities, and demineralization. Unfortunately less than one third of cases will have plain film evidence of osteomyelitis even after 1 week [7]. The majority of cases (90%) do not manifest plain film evidence of infection until 1 to 4 weeks (radiographs require a 30-50% loss of bone mineralization before a lucency may be depicted) [7,41]. CT may show increased density within the marrow, but this may be difficult to appreciate. On MRI, replacement of marrow fat with edema (water) and exudate results in a decreased signal on T1 and an increased signal on T2 weighted images. This finding is not specific for osteomyelitis, however, and can also be found in tumor, acute infarction, or fracture. The overall sensitivity and specificity of MRI for the detection of acute osteomyelitis ranges from 92-100%, and 89-100% respectively. MRI may be particularly useful in evaluation of the spine, where osteomyelitis shows a characteristic pattern of confluent vertebral body and disk involvement.
Technetium-MDP Bone Scan in Osteomyelitis
Bone scan is highly sensitive for the diagnosis of osteomyelitis
and bone scintigraphy can detect osteomyelitis 1 to 2 weeks before
radiologic changes are manifested (bone scans imaging are often
rapidly positive within 24-48 hours of the onset of symptoms)
[7,41]. Even a 5% change in bone turnover can be detected at bone
scintigraphy [41]. Unfortunately, the exam is non-specific as any
insult to bone may produce an abnormality.
For the detcetion of osteomyelitis, a 3-phase bone scan is performed consisting of an angiographic flow phase, a blood pool phase, and a delayed imaging set 2 to 4 hours later. The 3 phase bone scan has a reported sensitivity of 90-100% and a specificity of 70-95% for identification of osteomyelitis in non-violated bone. Specificity drops to about 35% in patients with complicating bone conditions (trauma, orthopedic hardware, etc...). In acute osteomyelitis a focal area of increased osseous activity is identified on all 3-phases of the study. In an adult a negative bone scan essentially rules out infection [6,7]. Bone scans may remain positive on delayed images for up to 1 to 2 years following successful treatment of osteomyelitis due to continued remodeling. 67Ga imaging is a better technique than either bone or white blood cell imaging when assessing response to treatment. It is most useful to have a baseline 67Ga scan at the initiation of therapy as a baseline. Return of the 67Ga activity to normal or very near normal signifies a complete response to antibiotic therapy based on studies in the rabbit model.
Osteomyelitis in children: Because symptoms can be poorly localizing and infection multifocal it is important to performed whole body imaging. Subperiosteal pus/edema, joint effusion, or vasospasm can produce small vessel occlusion which is sufficient to reduce blood flow to the infected area [5,7]. Therefore, in a child, hyperemia may be absent, even when there is a clear abnormality on delayed images [7]. High quality images of the metaphyseal/epiphyseal regions are particularly important, since subtle asymmetric activity may be the only indicator of an early osteomyelitis [7]. Photopenic (cold) defects on delayed images are uncommon, but may be seen particularly in the very early stages of the infection [7] and may reflect decreased tracer delivery due to internal bone pressure, vascular thrombosis, or bone infarct [41]. False negative exams are unusual, but they may occur in the transition period from decreased to increased tracer localization [5]. Thus in children, even if the bone scan is normal, if there is sufficient clinical suspicion, a repeat exam should be performed in 2 to 3 days, or a gallium exam may be performed. As the condition evolves into the subacute phase, hyperemia rather than ischemia becomes the dominant finding.
|
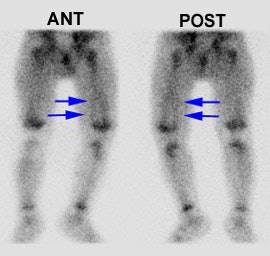
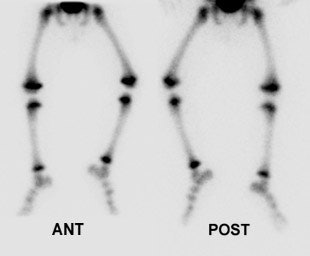
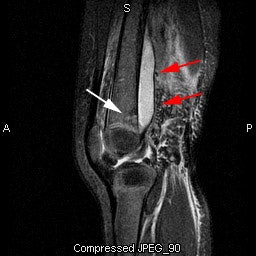
Pediatric osteomyelitis: The case below is from a 19 month old infant who presented with left leg pain. The leg was externally rotated and initially the child was thought to have a septic hip- however, a hip ultrasound was negative. A bone scan was ordered to evaluate for osteomyelitis. The flow images showed mild hyperemia in the left distal femur (click here to view flow images), but there was an elliptical area of abnormal activity on blood pool images involving the distal left femur (blue arrows). The delayed images did not reveal a significant focal finding and were interpreted as negative. The blood pool abnormality was felt to be related to a soft tissue infection. Because of a high degree of concern, an MR exam was then performed. The MR demonstrated a large subperiosteal pus collection (red arrows) and a small focus of osteomyelitis in the distal left femoral metaphysis (white arrow). This is an example of a false-negative bone scan in pediatric osteomyelitis. The late phase bone scan images may have been negative due to the small size of the focus of infection or due to imaging during the transition from "cold" to "hot" osteomyelitis. |
|
|
Cellulits vs osteomyelitis: Cellulitis can be differentiated from osteomyelitis as it typically demonstrates diffuse increased activity in the soft tissues on the dynamic and blood pool images, with no focal osseous abnormality on delayed images [7]. Mild increased activity in the bone subjacent to the cellulitis may be seen due to the increased flow to the area. In order to better differentiate cellulitis from osteomyelitis, delayed imaging at 24 hours can increase the specificity of the examination to 80-85% particularly in patients with peripheral vascular disease. Areas of involvement with osteomyelitis generally show increased intensity on the 24 hour image due to better clearance of the soft tissue activity. Thus, delayed images improve target (bone) to background (soft tissue) activity.
Gallium-67 Scan in Osteomyelitis:
The addition of Gallium imaging to conventional bone scintigraphy is also reported to increase specificity, and to provide a slight improvement in sensitivity. The gallium scan can be positive as early as 4 hours after the onset of infection, and it is usually positive by 24 hours [8]. Unfortunately gallium imaging is hindered by the same lack of specificity as conventional bone scintigraphy since gallium localizes to bone as a calcium analog and is incorporated into the calcium hydroxyapatite crystal. It also localizes to bone marrow because of its behavior as an iron analog. The criteria for a positive gallium scan for osteomyelitis include: A.) Gallium uptake is greater in intensity than the uptake on the bone scan (the contralateral normal side is used to determine the degree of intensity of the abnormality) and/or B) The pattern of gallium uptake is disparate (incongruent or more extensive) from the pattern of uptake on the bone scan [7]. Congruent distribution of gallium activity in comparison to the bone scan in terms of intensity and spatial distribution is considered equivocal for osteomyelitis.
Tumeh [9] feels that a pattern of gallium uptake greater than the activity noted on the abnormal bone scan is the only reliable indicator of active osteomyelitis. Unfortunately, this pattern is present in only about 25% of patients with active osteomyelitis. Other authors have also concluded that regardless of the bone scan findings (increased, normal, or decreased activity), increased gallium activity greater than that of the bone scan is consistent with osteomyelitis, even if the gallium abnormality is smaller than that identified on the bone scan [10].
A congruent distribution pattern (i.e.: identical size) of gallium and Tc-MDP coupled with low Ga-67 accumulation is most commonly seen with healing bone. However this finding may occasionally be associated with chronic low-grade osteomyelitis. The inability to accurately distinguish between activity in the bone and adjacent soft tissue or joints may lead to a false-positive exam. Nonetheless, a normal Gallium scan virtually excludes the diagnosis of acute osteomyelitis with a high degree of certainty (negative predictive value 90-100%) [11].
Gallium is also effective for identifying osteomyelitis in neonates and children due to better identification of lesions adjacent to the growth plates. In this setting, a Gallium exam should be used when the bone scan is inconclusive or shows a cold defect. 111In-WBC imaging is not commonly performed in children due to the high radiation exposure to the spleen and the potential for more mutagenic effects on lymphocytes given the expected longer life span of the child. 99mTc-HMPAO labeled leukocytes may be used in this setting.
Following successful treatment, uptake of Gallium should decrease even if the bone scan remains abnormal. Gallium activity frequently does not completely return to normal, but this should not be considered indicative of chronic osteomyelitis. If the Gallium scan does return to normal, a cure can be predicted with high reliability.
In-111 WBC Scan in Osteomyelitis
In-111 labeled WBC's provide a highly sensitive and specific exam for the evaluation of osteomyelitis. 111In WBC imaging has a higher sensitivity and specificity for the diagnosis of acute osteomyelitis than combined bone/Gallium imaging [7]. Schauwecker [12] noted In-111 WBC imaging to have a sensitivity of between 90-95% in acute osteomyelitis for all bones in the body (overall sensitivity from various studies ranges from 80 to 100% [7]). In-111 WBC imaging has a higher sensitivity for detecting acute osteomyelitis than it does for chronic osteomyelitis and this seems to be the most prevalent opinion [13]. For chronic osteomyelitis, the sensitivity of In-111 WBC imaging varied by location: Peripheral skeleton-94%, Axial skeleton-53%. This may be because chronic osteomyelitis is characterized by infiltration with predominantly mononuclear cells, versus the predominant neutrophilic infiltration seen in acute osteomyelitis and mixed leukocyte cell preparations contain fewer monocytes.
Because of the low dose of the agent used, it may be difficult to localize In-111 WBC activity to bone versus neighboring soft tissues [7]. An accompanying bone scan can often serve as a "road map" and aid greatly in making this distinction [7].
Non-specific uptake of the agent in the bone marrow can result in a false-positive interpretation [7]. Generally, marrow activity is symmetric, but it can be variable [7]. When in doubt, a bone marrow scan with Tc-sulfur colloid can aid in exam interpretation [7]. Uncommonly (up to 12% of cases), osteomyelitis may appear as a photopenic (cold) defect on In-111 WBC scintigraphy [7]. This occurs more commonly in areas with significant red marrow where uptake in the marrow is higher than in inflammatory site [7]. Ga-67 imaging may be beneficial in these cases [7].
Bone scans of injured bone are much more difficult to interpret. Reactive bone due to a fracture, hypertrophic non-union, and pseudoarthroses all demonstrate intense 99mTc-MDP and Gallium uptake. Similarly, the placement of orthopedic hardware such as a metal plate will result in increased uptake of both Gallium and 99mTc-MDP for at least 6 months. If the pattern of Gallium distribution is different from that on the bone scan, or the uptake of Gallium more intense, infection should be strongly considered. Although 111In WBC imaging may also be more difficult to interpret in the setting of underlying bone pathology, it is felt to be superior to bone/Gallium imaging (which has an accuracy of 40%) in the detection of infection at sites of fracture non-union (WBC sensitivity 84%, specificity 72%, but up to 97% if activity is definitively localized to the bone, and an accuracy of 88%). Mild to moderate WBC accumulation within uninfected callous formation may be difficult to distinguish from infection. Unexpected ectopic bone marrow may occur in the appendicular skeleton after trauma and repeated surgical interventions [31,38]. Tc sulfur-colloid marrow imaging is often useful in these cases.
Bone Marrow Scintigraphy in Osteomyelitis:
A marrow defect may not be appreciated for 5 to 6 days after onset of infection on bone marrow scintigraphy of acute osteomyelitis. This can be useful in the differentiation of bone infarction from infection in sickle cell patients who present with acute symptoms (acute infarction will produce a marrow defect on marrow scintigraphy and WBC imaging) [36]. In contrast, normal uptake on marrow images with abnormally high uptake on WBC imaging is indicative of osteomyelitis [36]. The Tc-SC preparation used for the exam should be less than 2 hours old (visualization of bladder activity indicates breakdown of the agent) [38].
Unexpected ectopic hematopoietic marrow activity can be seen in the appendicular skeleton following trauma and in patients with repeated surgical interventions. Tc-99m-SC marrow scinitgraphy can aid in differentiating osteomyelitis from non-infectious In-111 WBC marrow activity in these cases [25]. The pathophysiologic changes responsible for conversion of peripheral fatty marrow into hematopoietically active marrow have not been fully described, but may be related to osseous reparative action [16]. Marrow activity can also occur in the region of Charcot joints and may contribute to the accumulation of labeled WBC's [16].
In-111 WBC Imaging in the Neuropathic/Charcot Joint:
About 5% of patients with diabetes mellitus complicated by peripheral neuropathy will develop a neuropathic joint, most commonly involving the tarso-metatarsal joints (60% of cases), the metatarsal phalangeal joints (30% of cases), and the tibiotalar joints (10% of cases) [38].. Although superinfection of a neuropathic joint is uncommon, differentiating rapidly progressive neuropathic change from infection on radiographs is difficult. Due to the destruction and new bone formation associated with a Charcot (neuropathic) joint, Tc-MDP bone scintigraphy is also of limited use as the scan will appear "hot" on all three-phases [7]. Similarly, the gallium scan can also be expected to demonstrate increased activity. 111In labeled leukocytes do not usually accumulate in areas of increased bone turnover (neuropathic bones), however, false-positive results have been reported in up to 50% of neuropathic joints. It is believed that hematopoietically active marrow in the region of the neuropathic change contributes to the accumulation of labeled WBC's [38]. The pathophysiologic changes responsible for conversion of peripheral fatty marrow into hematopoietically active marrow have not been fully described, but may be related to osseous reparative action. Combined In-111 WBC and Tc-sulfur colloid marrow imaging is the procedure of choice for the evaluation of patients with Charcot joints and as it can better identify those patients with true osteomyelitis. [16] WBC imaging at 4 and 24 hours following injection can also be useful in distinguishing infection from non-infectious neuropathic tracer accumulation. Neuropathic arthropathy will show decreasing tracer activity over time, while accumulation of WBC's within osteomyelitis will increase in intensity between 4 and 24 hours [15]. Another point to remember is that neuropathic changes are usually bilateral and any degree of asymmetry in WBC activity should be considered suspicious for osteomyelitis in this setting [7]. Image interpretation can be complicated by WBC accumulation within a foot ulcer which is difficult to separate from the bone.
Overall, the 111In WBC exam has a sensitivity between 73-89%, and a specificity between 69-91% in the evaluation of the diabetic foot. MRI has a sensitivity of about 82-89% and a specificity of 80-84% in this setting [14].
In-111 WBC Imaging in Vertebral Osteomyelitis/Discitis:
Discitis:
There is a higher incidence of discitis in children with peaks seen between the ages of 1 to 4, and 12 to 15 years [30]. In young children, the higher incidence is felt to be related to the vascular supply of the spine. In children subchondral vessels are present which originate in the vertebral body that supply the disc [30]. This pathway is usually present until age 8, but may persist into teenage years [30]. In adults the disc is relatively avascular which makes hematogenous invasion less likely [30]. Positive blood cultures are obtained in less than 50% of cases of discitis. The infection is typically indolent [30]. The most common sites are the lower lumbar spine in children and the lower thoracic spine in teenagers.
The first radiographic indication of disc infection is loss of disc height. Irregularity and erosion of the adjacent vertebral end-plates appears later. Bulging of the paraspinal lines may also be seen. MRI is particularly sensitive to the changes in the signal intensity of the disc even early in the infection. Ordinarily the nucleus pulposus has a higher signal intensity than the annulus on T2 images because of its water content. In children the involved disc will demonstrate decreased signal intensity on both T1 and T2 images In adults decreased signal is seen on T1 images, with increased signal on T2 images. Paravertebral extension is also easily identified.
On scintigraphy in discitis delayed images will demonstrate increased tracer activity in the disc space and the contiguous vertebral bodies [30]. Blood pool images may also demonstrate increased tracer activity.
If the bone scan is normal, but the clinical suspicion is high, Gallium imaging may be helpful. The gallium exam is usually positive within 24 hours of injection. A characteristic "butterfly" pattern of tracer accumulation can be seen in both discitis and vertebral osteomyelitis. Because scintigraphy cannot identify the presence of an epidural abscess which may complicate diskitis, an MRI is recommended in all cases [5].
Vertebral Osteomyelitis:
The most common sources for vertebral osteomyelitis are infections of the urinary, skin, or respiratory system [30]. The most common organisms are S. aureus and E. coli [30]. Vertebral osteomyelitis has a bimodal incidence- with one peak in early childhood and another in the 6th decade [43]. In children, the infection typically involves the lumbar spine and often produces systemic complaints such as fever and irritability [30]. Abdominal pain, anorexia, and ileus may also be seen.
The earliest radiographic finding of vertebral osteomyelitis is rarefaction of the bone in the regions adjacent to the end-plates. This finding is usually not seen before 6 to 10 weeks. Findings similar to discitis may also be seen.
Data indicates that 111In leukocyte imaging is not as sensitive for infection of the spine as for other musculoskeletal infections and may be falsely negative in up to 80% of cases. The difficulty in interpretation may be related to the large percentage of spinal osteomyelitis which produces a cold, rather than a hot lesion (marrow uptake in the spine may be higher than in the adjacent inflammatory site which may mask the abnormality or cause the appearance of a cold defect). The duration of patient symptoms affects the likelihood of a vertebral osteomyelitis presenting as a cold defect. Most patients with symptoms less than 1 month demonstrate increased uptake, while a photopenic defect is more common in those with symptoms over 1 month [17]. MR imaging is probably the modality of choice when evaluating patients for suspected vertebral osteomyelitis. In patients that cannot undergo an MR exam, then bone-gallium imaging should be performed (overall accuracy is between 65-80%).
|
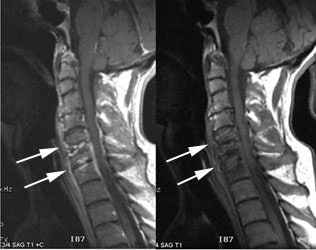
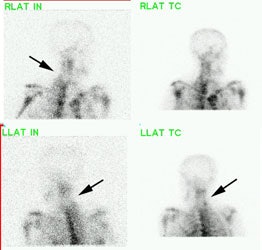
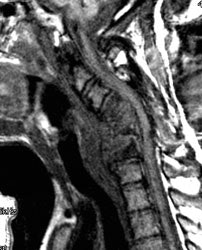
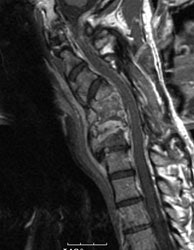
Cervical spine discitis and osteomyelitis: The case below is an example of the decreased sensitivity of In-111 WBC imaging for spinal infection. The MR images show abnormal disc enhancement at the C6-7 level consistent with discitis and enhancement of the C7 vertebral body consistent with osteomyelitis (white arrows). Post-gadolinium images are on the left. An In-111 WBC and Tc99m-Sulfur colloid exam in the same patient demonstrates a photopenic defect (black arrows) corresponding to the area of infection confirmed on the MR exam (white blood cell images are on the left). A defect on Tc-sulfur colloid imaging is seen with osseous infection due to marrow infiltration with inflammatory cells and edema which obstructs small arterioles. Click images to enlarge. |
|
|
|
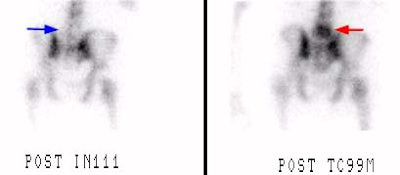
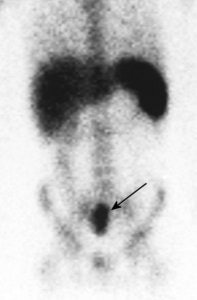
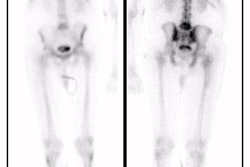
L5 vertebral osteomyelitis: The case below also illustrates the decreased sensitivity of In-111 WBC imaging for the detection of vertebral osteomyelitis. Bone scan revealed increased tracer activity within L5 (red arrow) in a patient with osteomyelitis at this level. The In-111 WBC exam demonstrated no evidence of increased tracer accumulation in L5 (blue arrow). Case submitted by Dr. Marc Cote, D.O. |
|
|
|
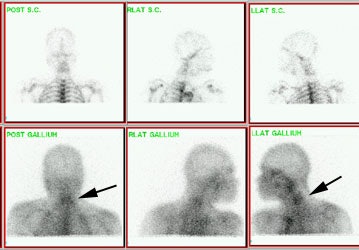
Gallium imaging for vertebral osteomyelitis: The patient below had an extensive cervical spine osteomyelitis involving C4 to C6. A pre-contrast MR image is shown on the left (degraded by motion), and a post-gadolinium image is shown in the center. Gallium-67 imaging revealed increased tracer activity corresponding to the area of abnormality on MR (black arrow) and was going to be used to monitor response to therapy. SPECT imaging would have been beneficial, but the patient could not tolerate the exam. Note the photopenic defect in the area of infection on corresponding Tc99m-sulfur colloid images (top row of nuclear medicine images). |
|
|
|
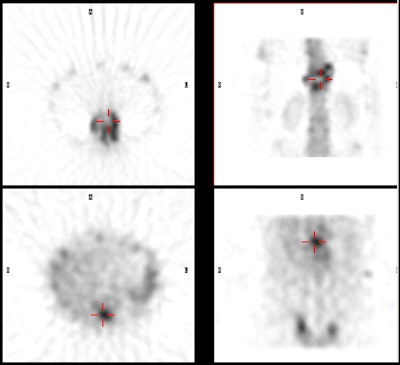
Spinal osteomyelitis: The patient
below was being evaluated for possible spinal
osteomyelitis. The SPECT bone scan (top images)
demonstrated an area of abnormal tracer uptake in the
lower thoracic spine with a cenrtal area of photopenia
(crosshairs). The SPECT gallium images (below)
demonstrated focal gallium concentration in the region of
photopenia- a pattern that was incongruent to the bone
scan and consistent with osteomyelitis. |
|
|
Cold lesions in the spine on In-111 WBC imaging are also not
specific for infection and can be seen secondary to neoplasm
(metastatic disease), Paget's, avascular necrosis, radiation
therapy, fracture, myelofibrosis, and fibrous dysplasia. [18]
|
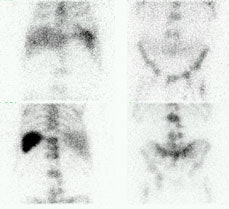
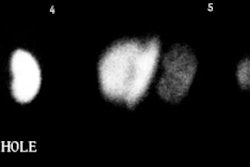
Metastatic prostate cancer on In-111 WBC imaging: The patient below was being evaluated for a fever of unknown origin. The WBC study revealed photopenic lesions involving the spine due to prostate metastases. |
|
|
In-111 WBC Imaging of Endoprostheses:
(Discussed separately in post-operative joint evaluation section)
In-111 WBC in Abdominal Abscesses:
(Sensitivity 88%, Specificity 90%)
111In WBC scans are superior to Gallium for evaluation of suspected abdominal-pelvic abscess due to the lack of a normal bowel excretory pathway. Some abscesses accumulate 111In WBC's very slowly, and 48 hour delayed imaging may be necessary to identify these lesions. Evaluation of the upper abdomen may be enhanced by the addition of a Tc-sulfur colloid scan and subtraction of liver and spleen activity from the 111In image. Labeled WBC's accumulate in the pancreas only in cases of "severe" pancreatitis and in infected pseudocysts. Sterile pseudocysts have not been reported to show increased leukocyte accumulation. In appendicitis a focal area of increased activity in the right lower quadrant may be identified. This finding may also be seen with an inflamed or perforated Meckel's diverticulum or a tubo-ovarian abscess.
In-111 WBC's for Inflammatory Bowel Disease:
111In WBC's can be used to assess for disease activity and distribution. Excellent correlation is found between endoscopy, histology, and 111In WBC scans for disease extent and activity. When using 111In WBC's for imaging of inflammatory bowel disease, early imaging (1-2 hours) is required as labeled cells sloughed from sites of active inflammation can be carried distally causing the appearance of pan-colitis, multiple sites of bowel inflammation, etc. Tc-99m-HMPAO labeled WBC's can also be used for detection of areas of active inflammation, but images should be acquired at 1 hour post-injection as later images (after 3 hours) may demonstrate non-specific bowel activity due to biliary excretion of secondary hydrophilic complexes [26,35]. These complexes are also excreted in the urine which results in bladder activity that may obscure pelvic disease [35].
Fecal collection may also be performed. In normal individuals no more than 2% of the injected dose is excreted in the feces. Fecal excretions of up to 60% can be seen in patients with inflammatory bowel disease.
Other causes of bowel/abdominal accumulation of radiolabeled WBC's must be excluded such as: Neoplasm (approximately 12-34% of tumors demonstrate 111In WBC uptake and this may be related to the presence of an inflammatory reaction around the tumor), bowel infarction, GI bleeding, pseudomembranous colitis/ infectious colitis, intra-abdominal hematoma, phlegmon, accessory spleens, and swallowed activity from pulmonary or sinus infections. For the latter reason, images of the head, neck and chest region are recommended whenever bowel activity is seen on abdominal imaging.
|
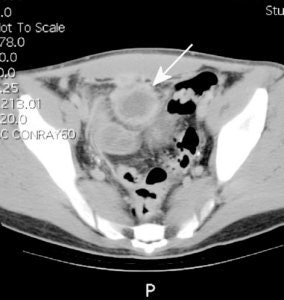
Crohns disease with abscess: The patient below had Crohns disease and was being evaluated for persistent fevers and elevated white blood cell count. The In111-WBC exam demonstrated a very intense focal accumulation of labeled WBC's in the mid pelvis (black arrow). Large bowel activity can also be seen, however, these images were performed at 24 hours following injection and cells sloughed from sites of active inflammation can be carried distally causing the appearance of colitis. The CT scan (right) had actually been performed prior to the white blood cell study and had been interpreted as negative for abscess. In retrospect, the abscess can be seen on the CT scan (white arrow), but was missed because of poor bowel opacification. Click CT to view more images. |
|
|
In-111 WBC Vascular Imaging:
Prosthetic Valvular Heart Disease:
111In labeled WBC's can easily miss small vegetation's and lack appropriate physical characteristics for SPECT imaging limiting this techniques usefulness in the evaluation of infected valvular prostheses.
Vascular graft infection:
Vascular graft infection: Infection occurs in only 2% of patients [24], but is associated with a very high mortality ranging from 25 to 75% [28]. Standard treatment is surgical removal combined with extra-anastomotic bypass or in-situ replacement of the graft (in cases of minimal infection) and antibiotic therapy- but even this aggressive treatment can be associated with high mortality and morbidity [24]. Percutaneous drainage of infected grafts has also been successfully performed [24]. The most common offending organism is S. aureus. CT findings of graft infection include thickening of the graft wall or increased perigraft soft tissue, perigraft fluid, anastomotic pseudoaneurysm, or gas in the graft bed. Unfortunately, other than for the presence of gas in the graft bed, these findings are all non-specific. In the evaluation of infected prosthetic vascular grafts 111In WBC's have a sensitivity of 98-100%, and a specificity of about 85-89%. The examination may also provide useful information about the presence of extragraft sites of infection. Scans performed within 1 week of surgery may demonstrate uptake of labeled cells in the graft, but by 1 month post-op an uncomplicated graft should not be visualized. False-positive WBC images may occur due to: Active bleeding at the site of the aneurysm or hematoma, a lymphocele [27], active thrombus formation at the site of the aneurysm due to accumulation of radiolabeled platelets and red cells - (which is generally NOT a problem if heparin is used as the anticoagulant during cell labeling as opposed to ACD), or a non-infected post-surgical pseudoaneurysm (also due to incorporation of labeled platelets into thrombus) [21]. Pseudoaneurysm formation is associated with infection in about 10% of cases [20,21].
Tc-HMPAO labeled WBC's have also been used in the evaluation of graft infections [28]. Flow, 5 minute, 30 minute, and 3 hour images should be obtained over the region of concern. Whole body images should also be acquired at 3 hours. Occasionally 24 hour images are helpful. Fasting the patient following injection of the labeled WBC's helps to decrease bowel activity secondary to hepatobiliary excretion of Tc-HMPAO. The HMPAO exam has been reported to have a sensitivity of 100% and a specificity between 92-100%. Infected grafts tend to show only mild uptake of tracer on the early images, but significantly increased activity on the 3 hour images. False positive scans have been reported particularly in the early post-operative period (within one month following surgery) most likely secondary to post-surgical perigraft inflammation. However, uptake of the tracer in non-infected grafts can be seen for up to 3 months following surgery [22].
FDG imaging has also been used to identify graft infection.
Pseudoaneurysms appear as hot spots on the flow study, but decrease in intensity over time, and significant pseudoaneurysm uptake is never identified at 3 hours. [23]
In-111 WBC Pulmonary Uptake:
Pulmonary uptake of leukocytes is presumably due to non-specific inflammatory responses that stimulate leukocyte accumulation in the lungs through increased vascular permeability due to complement activation, endothelial damage, or pulmonary vascular bed vasodilatation.
Focal pulmonary uptake is associated with infection only 50% of the time. Focal uptake that is segmental or lobar in appearance is most often associated with bacteria pneumonia (positive predictive value 92%) [34]. Focal uptake may also be seen with atelectasis and rarely with pulmonary embolism or infarction. Multiple, bilateral, small foci of activity can be seen secondary to clumping of the labeled white blood cells and should not be equated with septic emboli [34].
Diffuse pulmonary uptake is seen in 15-20% of patients, is relatively nonspecific, and is associated with infection in only 10% of cases. Gallium is probably the agent of choice for the evaluation of pulmonary inflammatory abnormalities. The differential of diffuse pulmonary uptake of 111In WBC's includes:
Infection:
- Opportunistic infections such as pneumocystis carinii pneumonia, CMV pneumonitis, or Mycobacterium avium-intracellulare
Non-infectious:
- Congestive heart failure
- Adult respiratory distress syndrome: Due to increased permeability of the alveolar-capillary membrane resulting in migration of leukocytes into the lung parenchyma [34].
- Drug induced pneumonitis: Induces an intense neutrophilic reaction in the lungs
- Aspiration
- Following XRT
- Following cardiopulmonary resuscitation
- Following hemodialysis: Complement activation after hemodialysis results in increased pulmonary sequestration of leukocytes [34].
- Normal uptake: Images obtained shortly after injection of labeled cells demonstrate diffuse pulmonary activity which decreases over time until about 4 hours after injection [34]. This finding is likely related to slow passage of labeled white blood cells through the pulmonary capillary bed [34].
|
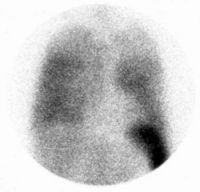
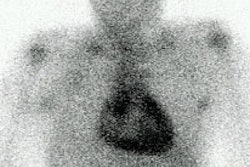
Non-specific diffuse lung uptake: Diffuse lung activity can be seen in this asymptomatic patient. Diffuse lung activity is uncommonly associated with infection. |
|
|
False-Positive In-111 WBC Examinations [7]:
- Bone marrow activity misinterpretated as infection
- Sterile hematomas, bleeding wounds, and wounds healing by secondary intention
- Uninfected post-surgical wounds: Commonly show faint uptake for up to 10 days
- Renal transplants: Uptake can be seen normally and does not indicate rejection, infection, or ATN
- Stomas: Recent stomas may demonstrate modest uptake localized to the abdominal wall
- Intramuscular injection sites
- Gastrointestinal activity:
- Ischemic bowel
- Swallowed activity from sinus, gingival or pulmonary infection
- Tumors: Up to 34% of tumors with no evidence of infection may localize In-111 WBC's [7]. Uptake is particularly common in histiocytic lymphoma and skeletal metastases [7]. This may be related to an associated inflammatory reaction to the tumor, tumor necrosis, or to the presence of free In-111 which is tumoriphilic.
- Cerebral infarction
- Foci of increased tracer activity are also commonly seen at: Tracheotomy sites, NG tube sites, and G-tube sites
|
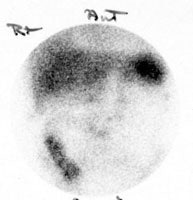
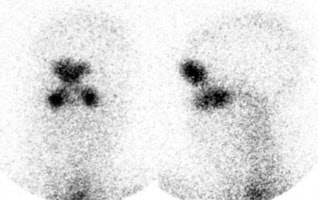
False-positive In111-WBC exam: Scanning over the abdomen (left image) in the patient shown below revealed abnormal bowel activity suggestive of inflammation or infection. However, the patient had severe pansinusitis (right two images are performed over the head) and the bowel activity was related to swallowed cells. |
|
|
REFERENCES:
(1) Nuclear Medicine Annual 1994: Palestro CJ. Musculoskeletal infection. Ed. Leonard Freeman. Raven Press, Ltd, New York. 91-119
(2) Datz and Thorne
(3) Nuclear Medicine Annual 1993: Datz FL. The current status of radionuclide infection imaging. Ed. Leonard Freeman. Raven Press, Ltd, New York. 47-76
(4) AJR 1986; Williamson MR. 111 In-labeled leukocytes in the detection of prosthetic graft infections. 147: 173-176
(5) J Nucl Med 1998; Connolly LP, et al. Assessing the limping child with skeletal scintigraphy. 39: 1056-1061
(6) Radiology 1985; Al-Sheikh W, et al. Subacute and chronic bone infections: Diagnosis using In-111, Ga-67 and Tc-99m MDP bone scintigraphy, and radiography. 155: 501-506
(7) Ortho Clin N Am 1991; Wegener WA, Alavi A. Diagnostic imaging of musculoskeletal infection. Roentgenography; gallium, indium-labeled white blood cell, gammaglobulin, bone scintigraphy; and MRI. 22: 401-417
(8) Alazraki N. Musculoskeletal imaging. In: Taylor A, ed. Clinical Practice of Nuclear Medicine. New York: Chirchill-Livingstone, 1991: 379-431 (p.414)
(9) Tumeh, Radiology, 1986
(10) Radiology 1984; Dec: p. 808
(11) Nuclear Medicine Annual 1994; Palestro CJ. Musculoskeletal infection. Ed. Freeman LM. Raven Press, Ltd. New York. 91-119
(12) Schauwecker, Radiology 1989
(13) Radiologic Clinics North America 1993; July
(14) Radiology 1996; Aug: p.557-564
(15) J Nucl Med 1995; Schauwecker DS. Differentiation of infected from noninfected rapidly progressive neuropathic osteoarthropathy. 36: 1427-28
(16) J Nucl Med 1998; Palestro CJ, et al. Marrow versus infection in the Charcot joint: In-111 leukocyte and technetium-99m-sulfur colloid scintigraphy. 39: 346-350
(17) Nuclear Medicine Annual 1994; Palestro CJ. Musculoskeletal infection. Ed. Freeman LM. Raven Press. N.Y. 91-119
(18) Nuclear Medicine Annual 1993; Datz FL. The current status of radionuclide infection imaging. Ed. Freeman LM. Raven Press, Ltd. New York. 47-76
(19) J Nucl Med 1989; Oswald SG, et al. Three-phase bone scan and indium white blood cell scintigraphy following porous coated hip arthroplasty: a prospective study of the prosthetic tip. 30: 1321-31
(20) AJR 1986;147 July: p.173-76
(21) Radiology 1986; Indium-111 labeled leukocyte uptake: False-positive results in non-infected pseudoaneurysms. 158: 761-63
(22) J Nucl Med 1998; Liberatore M, et al. Clinical usefulness of technetium-99m-HMPAO-labeled leukocyte scan in prosthetic vascular graft infection. 39: 875-879
(23) J Nucl Med 1994; Prats E, et al. Diagnosis of prosthetic vascular graft infection by technetium-99m-HMPAO labeled leukocytes. 35: 1303-07
(24) AJR 1998; Belair M, et al. Aortic graft infection: The value of percutaneous drainage. 171: 119-124
(25) J Nucl Med 1998; Kaim A, et al. Ectopic hematopoietic bone marrow activity in the appendicular skeleton after trauma. 39: 1980-1983
(26) J Nucl Med 1993; Arndt JW, et al. Prospective comparison study of technetium-99m-WBCs and indium-111-granulocytes for the examination of patients with inflammatory bowel disease. 34: 1052-1057
(27) Clin Nucl Med 1992; Chung CJ, et al. Uptake of In-111 labeled leukocytes by lymphocele a cause of false-positive vascular graft infection. 17: 368-370
(28) Clin Nucl Med 1992; Mortelmans L. Clinical usefulness of Tc-99m HMPAO labeled white blood cell imaging in prosthetic vascular graft infections. 17: 11-13
(29) Semin Nucl Med 1993; Miller JH. The role of radionuclide-labeled cells in the diagnosis of abdominal disease in children. 23: 219-230
(30) Semin Nucl Med 1993; Sty JR, et al. Spine pain in children. 23: 296-320
(31) J Nucl Med 1998; Kaim A, et al. Ectopic hematopoietic bone marrow in the appendicular skeleton after trauma. 39: 1980-1983
(32) Radiographics 2001; Love C, et al. Role of nuclear medicine in diagnosis of the infected joint replacement. 21: 122901238
(33) Clin Nucl Med 1993; Belur S, et al. Skeletal photopenic lesions on Indium-111 WBC imaging; Differential diagnosis. 18: 439-440
(34) Radiographics 2002; Love C, et al. Pulmonary activity on labeled leukocyte images: physiologic, pathologic, and imaging correlation. 22: 1385-1393
(35) J Nucl Med 2004; Peacock K, et al. 99mTc-stannous colloid white blood cell scintigraphy in childhood inflammatory bowel disease. 45: 261-265
(36) Radiographics 2007; Ejindu VC, et al. Musculoskeletal manifestations of sickle cell disease. 27: 1005-1021
(37) J Nulc Med 2007; D'Alessandria C, Signore A. Diagnosis of vascular prosthesis infection: PET or SPECT? 48: 1227-1229
(38) Radiographics 2006; Palestro CJ, et al. Combined labeled leukocyte and technetium 99m sulfur colloid bone marrow imaging for diagnosing musculoskeltal infection. 26: 859-870
(39) J Nucl Med 2009; Fisher DR, et al. MIRD dose estimate report No. 20: radiation absorbed-dose estimates for 111In- and 90Y-ibritumomab tiuxetan. 50: 644-652
(40) J Nucl Med 2009; Palestro CJ. Radionuclide imaging of
infection: in search of the grail. 50: 671-673
(41) Radiographics 2012; DiPoce J, et al. Pediatric
osteomyelitis: a scintigraphic case-based review. 32: 865-878
(42) J Nucl Med 2016; Palestro CJ. Radionuclide imaging of
musculoskeletal infections: a review. 57: 1406-1412
(43) Radiology 2017; Jaramillo D, et al. Hematogenous
osteomyelitis in infants and children: imaging of a changing
disease. 283: 629-643
(44) AJR 2019; Sethi I, et al. Current status of molecular
imaging of infection: a primer. 213: 300-308